mAbs: the rising wave of innovations in veterinary medicine
Monoclonal antibody (mAb) therapies could revolutionize how veterinarians care for pets with chronic diseases.
This audio recording was enabled by AI
Key Points
- Monoclonal antibodies are designed to mimic pets’ naturally occurring antibodies and provide precise, species-specific treatment with potentially fewer side effects than traditional therapies.
- mAbs are already transforming the control of osteoarthritis pain and allergic skin disease in pets. Zoetis is exploring their potential for longer-acting treatments and for addressing unmet needs in other chronic conditions like kidney disease and cancer.
- Rigorous research and collaboration drive the development of safe, effective, and scalable mAb therapies.
Humans have access to all sorts of therapies that treat chronic diseases. Zoetis scientists believe animals should too.
“Chronic diseases are complex and challenging, but we’re tackling them with passion and scientific rigor,” said Catrina Stirling, Director, Regulatory Affairs and lead for Zoetis’ renal development program.
“We aim to deliver novel, monoclonal antibodies (mAbs) that meet significant unmet medical needs and have meaningful impact for pets, their owners and the veterinarians who care for them.”
Catrina Stirling, Director, Regulatory Affairs and lead for Zoetis’ renal development program
Targeting the troublemakers
Like humans, pets have built-in defenses, called antibodies, that help their bodies fight disease.
“The biology of dogs and cats is unique, and different drivers cause disease to evolve. The beauty of mAbs is that they are designed to mimic the dog or cat’s naturally occurring defenses,” said Scott Knauer, Vice President of Global Therapeutics. “They are engineered to only target and bind to the protein that results in disease progression and jeopardizes a pet’s quality and length of life.”
Physicians have been using mAbs to treat diseases in humans for more than 40 years.1 As of January 2025, more than 160 mAbs have been approved for use in human health,2 yet in animal health, only five mAbs are available. Three of them are Zoetis innovations. Current research investments at Zoetis include more than 50 mAb targets across five species.3
A pressing need
Osteoarthritis (OA) pain, skin allergies, kidney disease, cancer and cardiovascular disease are among the chronic diseases that affect pets around the world. Treatments are available in many countries to control OA pain and canine allergic itch. For example, almost a decade ago, Zoetis developed and introduced a mAb to provide long-lasting itch relief to dogs with allergic skin disease. More than 11 million U.S. dogs, along with others in more than 40 countries, have benefited from the therapy.5
But pets with renal or oncology issues are mostly treated with palliative care.
“Veterinarians are often faced with delivering heartbreaking news: ‘There’s nothing we can do but keep your pet as comfortable as possible.’ No pet owner wants to hear this. We’re trying to fix that by expanding the toolbox for veterinarians with newer therapeutics,” said Scott.
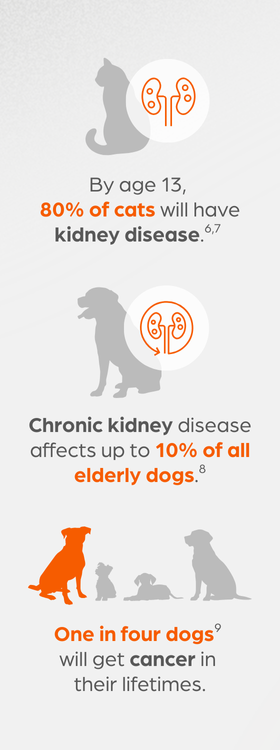
Consider renal disease: By age 13, 80% of cats will have kidney disease.6,7 Meanwhile, chronic kidney disease (CKD) affects up to 10% of all elderly dogs.8 Because there is no therapy to specifically target CKD, veterinarians can only offer palliative care, such as implementing diet restrictions and treating vomiting and dehydration.
Cancer is one of the most common causes of death in dogs and cats. For example, 1 in 4 dogs will get cancer9 in their lifetimes, and 50% of dogs over the age of 10 will get cancer.10 Surgery and chemotherapy are options, but both have drawbacks.
“Surgery is not always successful, and it’s very expensive,” said Amanda Guth, veterinarian, Research Director and head of the oncology program in Zoetis’ R&D organization. “Like all medications, chemotherapy can cause adverse effects for the animal. And without the proper equipment and training, chemotherapy and radiation can be toxic to veterinary staff in charge of administering the treatment.”
Access to specialized care is a challenge too. There are only about 600 board-certified veterinary oncologists in the U.S.,11 and in some countries, there are only a few.
“We continue to lead in the development of mAbs to give veterinary practices safe and effective treatment options for pets with chronic diseases,” said Scott. “The need for new therapies around the world is huge. We intend to help close that gap with the next wave of innovations.”
How could mAbs revolutionize veterinary care?
Scientists create mAbs in a lab by genetically modifying cell lines to express the monoclonal of interest targeting the right mediator or receptor. Because mAbs are designed to bind with high specificity to the disease-progressing culprits, they provide more precise treatment than traditional therapies.

“Traditional therapies are not always well tolerated. For example, NSAIDs have been used for years to control chronic OA pain in pets. Yet, they are poorly tolerated by cats and can only be used for a limited time because they can cause intestinal, kidney and liver issues,” said Monica Escalada, Director, Regulatory Affairs and project lead for a Zoetis’ OA pain development program in cats. “Monoclonal antibodies are targeted therapies that function like a naturally occurring antibody. They are metabolized and eliminated like naturally occurring antibodies, with minimal involvement of the liver or kidneys,12,13,14 which means lower risk of NSAID-like toxicity. Thanks to this, we can extend the treatment duration and, potentially, extend the pet’s life.”
Zoetis’ mAb development programs include a treatment for CKD in dogs that is intended to address fibrosis or scarring of the kidneys, a condition that leads to kidney failure. Conditional approvals for this novel therapeutic are anticipated in several markets in the next 12 to 36 months.
“The goal is to deliver a long-term mAb therapy that addresses disease progression and keeps the disease stable,” said Catrina. “Our hope is to deliver something new in the renal space that will have an impact on quality of life for the pet and pet owners too.”
To further advance OA pain treatment for dogs and cats and allergic and atopic dermatitis for dogs, scientists continue to explore longer-acting mAbs. “This could result in fewer visits to the vet’s office and better compliance of chronic disease management – and this could revolutionize veterinary medicine,” said Scott.

In oncology, scientists are creating mAbs that could help treat cancer, not just address symptoms, “whether that means killing cancer cells or invigorating the pet’s immune system, similar to recent cancer therapies in human health,” said Amanda.
Although mAbs could one day make pet care more convenient and precise, creating differentiated, longer-acting therapies is no simple task. The biology of each species is unique, and this complexity requires scientists to follow a rigorous and thorough R&D approach that begins with the unmet need.
Our meticulous approach to developing mAbs
Zoetis scientists have spent more than a decade researching and developing mAbs and other therapeutic solutions to help pets live healthier, longer. These innovations are possible because of a meticulous approach:
- Define the problem
This is the first step before any program begins. “We always start by asking, ‘What’s missing in veterinary medicine? What are the gaps in treatment, and what tools could help fill those gaps?’ From there, we focus our resources in areas where we can have the most impact and then marry the need with scientific curiosity,” said Scott. - Understand the animal’s biology and what’s causing the disease
Scientists set out to solve the mystery by gaining a deep understanding of the animal’s unique biology and what’s triggering a problem. Is it an overactive protein? A sluggish chemical? An out-of-whack receptor? A cytokine behaving badly? - Design mAbs to interact with the target
“The goal is to design a monoclonal antibody that is unique to a species and unique in its target,” said Scott. “A well-designed mAb will bind to the intended target and produce a desired effect, such as slowing down the progression of the disease, disrupting a signal or controlling pain,” said Scott.
But not just any design will work. “It must be functionally potent and safe for the animal and safe for the veterinary professional administering the therapy. A safety-mindset is a must for our teams,” said Amanda. “Also, the mAbs we design must be scalable – a therapy we can efficiently manufacture to meet demands and deliver cost-effectively for pet owners.” - Meet and exceed regulatory requirements
“Regulatory processes globally are robust. Safety and efficacy requirements for animal health therapies are very similar to those in human health,” said Monica. “From the beginning, a formal, regulated process is in place including protocols for how to conduct clinical trials. It’s a process our scientists are passionate about and even go beyond regulatory expectations to deliver safe and meaningful products to veterinary medicine. It’s the right thing to do.” - Bringing together the right experts and partners
To continue driving innovation in animal health, Zoetis scientists collaborate with multiple internal and external experts, from key opinion leaders to veterinary practices across the globe. The R&D organization also continues to add more scientists to development teams – biologists, protein engineers and other experts who work together to solve the complexities of chronic disease.
“We are committed to being the most trusted provider of veterinary mAbs, and the work we do gives us a deep sense of purpose,” said Scott. “Our scientists are also pet owners, and we are determined to continue generating and developing effective, safe therapeutics that we trust using on our own pets.”
Published on November 19, 2025
All trademarks are the property of Zoetis Services LLC or a related company or a licensor unless otherwise noted. © 2025 Zoetis Services LLC. All rights reserved. ZPC-04574R1
References
1 The history of monoclonal antibody development – Progress, remaining challenges and future innovations | PMC
2 Current Review of Monoclonal Antibody Therapeutics in Small Animal Medicine | MDPI
3 "Monoclonal Antibodies: Defining a New Category for Animal Care,” Zoetis 2023 Annual Report
4 The history of monoclonal antibody development – Progress, remaining challenges and future innovations | PMC
5 Kynetec PetTrak GAH-284 Unique Patient Count Since Launch Apoquel Cytopoint Jan 2014–June 2025
6 Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies | PubMed
7 Chronic Kidney Disease (CKD) in Dogs and Cats | The Animal Medical Center
8 IBID
9 Pet Owner Resources | Veterinary Cancer Society
10 Cancer in Pets | American Veterinary Medical Association
11 Veterinary specialists in the U.S. | American Veterinary Medical Association. December 31, 2024.
12 Librela (bedinvetmab injection). Freedom of Information Summary. NADA 141-562. Zoetis Inc; May 2023.
13 Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493-507. doi:10.2165/11531280
14 Olivry T, Bainbridge G. Advances in veterinary medicine: therapeutic monoclonal antibodies for companion animals. Zoetis. March 2015.